Major depression is one of the most common mental disorders in the United States, with an estimated 21 million U.S. adults experiencing a depressive episode in 2021. Medication and therapy are often used to treat depression, but they do not work for everyone.
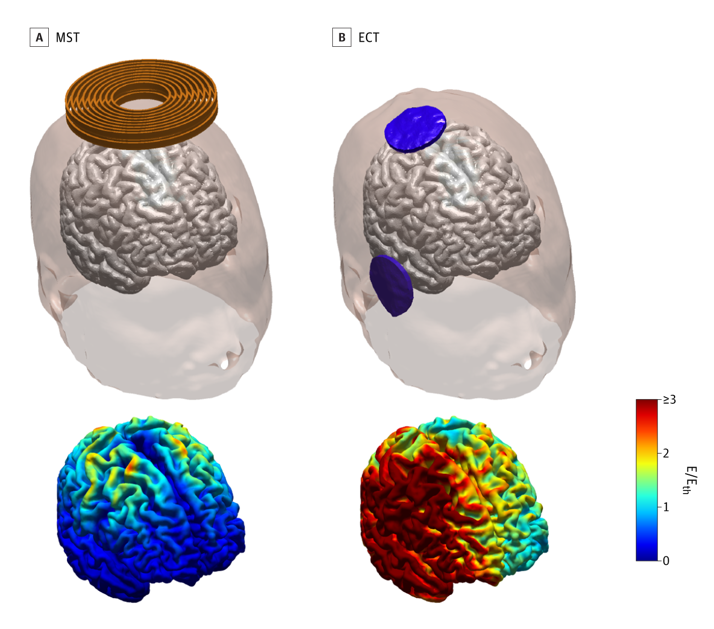
Electroconvulsive therapy (ECT) is one treatment that is used when people have not found relief from depression symptoms through other treatments. In ECT, an electric current is used to induce widespread seizure activity in the brain. The seizure leads to chemical changes in the brain that relieve depression symptoms. Although ECT is effective at reducing depression symptoms, some people experience memory loss after treatment, especially the loss of memories about their personal history (called autobiographical memories). Sometimes, these memory problems can be severe.
Magnetic seizure therapy (MST) is a newer treatment being studied for depression. It has been designed to have all the benefits—and fewer of the memory and cognitive side effects—seen with ECT. In MST, a magnetic coil is held against the scalp. The magnetic coil induces seizures in the brain that are much more localized and milder than those created during ECT.
What did the researchers do?
Sarah H. Lisanby, M.D., Director of the Noninvasive Neuromodulation Unit in the Experimental Therapeutics and Pathophysiology Branch at the National Institute of Mental Health, and colleagues tested the effectiveness of MST as compared to ECT for treating depression symptoms.
The study included 73 participants between the ages of 18 and 90 who had experienced a major depressive episode and had been referred for ECT treatment. Participants were randomly assigned to receive ECT (38 participants) or MST (35 participants) three times a week until they achieved remission or until their response to the treatment leveled off.
Clinicians assessed participants for depressive symptoms at the beginning of the study (before treatment), the morning of each treatment session, 24 to 72 hours after each treatment session, and twice per month for 2 months after treatment ended and once a month thereafter for the duration of the study.
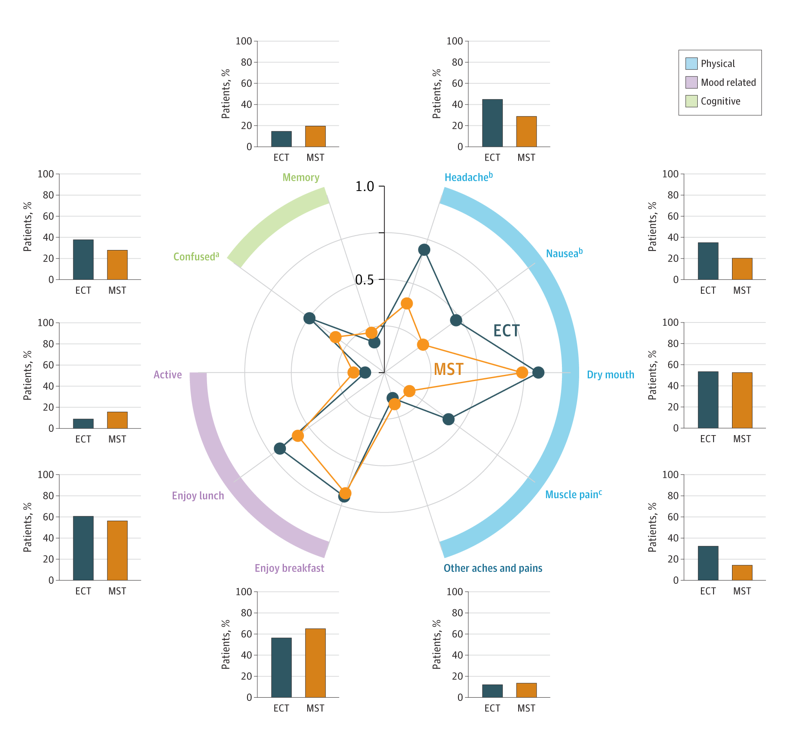
Clinicians also measured the cognitive abilities of patients at the beginning of the study and 72 hours after the last treatment session. After each treatment session, participants were asked to rate the presence and severity of possible side effects, such as headache, nausea, dry mouth, muscle pain, confusion, and memory problems.
What were the results of the clinical trial?
Among participants who completed the full course of treatment (24 for ECT and 29 for MST), 60.4% showed a significant decrease in depression symptoms, and 43.4% achieved depression remission. When the researchers looked at the impact of MST and ECT treatment on all participants in the study—those who completed some treatment combined with those who completed the full treatment—they found that 46.6% showed a significant decrease in depression symptoms and 31.5% achieved full depression remission.
The researchers found no significant differences in symptom reduction or remission between the ECT and MST groups, indicating the treatments were equally effective at relieving depression symptoms. Participants in the ECT group achieved depression remission slightly sooner (between 6 and 7 sessions) than the MST group (9 sessions). The antidepressant benefit of both treatments lasted up to 6 months after the last treatment session.
Patients receiving ECT reported more severe headaches, nausea, muscle pain, confusion, and disorientation than participants receiving MST. While global cognitive function remained intact for participants receiving either treatment, patients receiving MST showed greater recall and specificity of autobiographical memories and regained cognitive orientation after treatment faster than participants receiving ECT.
What do these findings mean?
The clinical trial found that MST is equally effective at reducing depression symptoms as ultrabrief right unilateral ECT—the safest form of ECT currently available. MST reduced depression symptoms for up to 6 months and had fewer side effects than ECT. The improved autobiographical memory performance and faster cognitive orientation seen following MST treatment suggest it provides a high level of cognitive safety for participants.
The findings demonstrate the promise of MST. Larger trials are currently underway to better understand the comparison between MST and ECT and to learn how to best optimize the delivery and dosing of MST.
Reference
Deng, Z.-D., Luber, B., McClintock, S. M., Weiner, R. D., Husain, M. N., Lisanby, S. H. (2023). Clinical outcomes of magnetic seizure therapy vs electroconvulsive therapy for major depressive episode: A randomized clinical trial. JAMA Psychiatry. https://doi.org/10.1001/jamapsychiatry.2023.4599




